Raising $5M in Series A Round for Commercialization and Sales
100 M stroke survivors globalally needing hand rehabilitation.
Current Standard-of-Care focusses on balance, gait and Speech
No effective Intervention for Hand = Life Long disability.
Costs ~$46 billion/Year to the US Economy

The MyHandTM System
Digital Neuro-Rehab Platform for Home or Clinic use
Large and poorly met market.
With a mission to help 100 million stroke survivors living with hand function disability. IRegained has developed the MyHand™ System – a neuroplasticity enhancing, research-driven, telemedicine-powered, clinically effective and affordable hand function rehabilitation system.

Consistent and repeated results from 3 independent studies conducted from 2021-2023
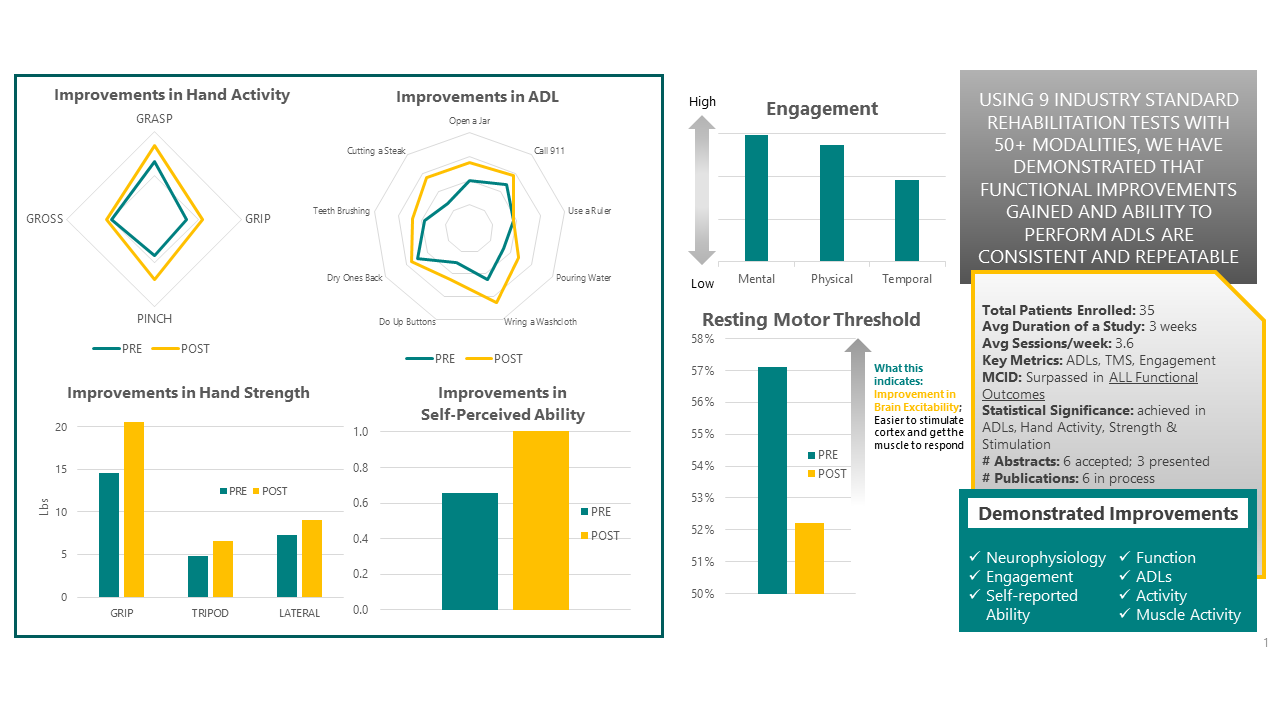
Key Highlights
Healthcare, science and engineering, technology, platforms, therapy, telehealth, software, medical devices, rehabilitation, stroke/neurology, musculoskeletal, neuroplasticity, telemedicine
Phase I: Rehabilitation Hospitals, Clinics, Nursing Homes, Skilled Nursing Facilities (SNFs), Long-term Care Centers and Individual PTs/OTs
Phase II: Home Healthcare Agencies and Individual Stroke Patients for Home-Use
In 70%, of stroke survivors, the brain damage from stroke results in impaired hand function. This makes mundane tasks, such as holding a cup, combing hair or toileting almost impossible to do.
Many stroke survivors have to live with this disability for rest of their lives, often times with the help of personal support workers and caregivers.
This can lead to a reduce overall quality of life for the patient, it also costs the US healthcare system $34 billion annually, without taking into account the lost workdays and ultimetly, the reduced economic output.
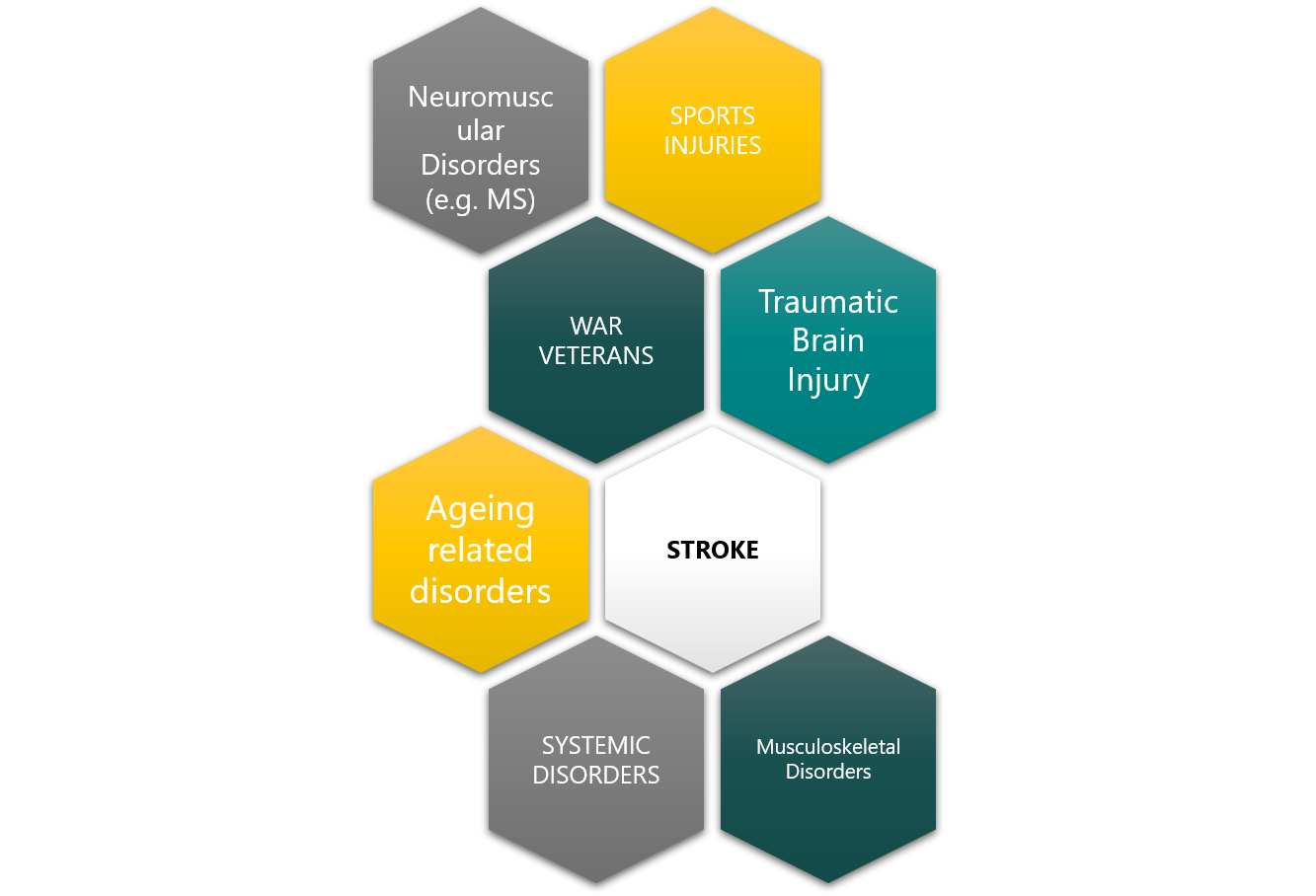
Beyond Stroke, hand function loss can happen from other conditions suc as, traumatic brain injury, musculoskeletal injury, war, industrial accidents or sports related injuries, in many cases leading to life-long disability and a very poor quality of life.
Recovering hand function requires targeted neuro-rehabilitation therapy with intensity and repetition for months and potentially years (10-20 sessions per week).
Furthermore, there are very few clinically validated hand-function rehabilitation devices in the market.
Those that exist are either ineffective (ex: squeeze balls, rubber bands, etc.) because they do not leverage neuroplasticity to recover control the hand, or cost prohibitive (e.g. robotic devices >$150,000) and therefore unaffordable.
The system leverages neuroplasticity, the human brain’s ability to ‘reprogram’ its neural pathways. Backed by 40 years of clinical and scientific research-based evidence and with guidance from accomplished rehab professionals, this system can help people with hand-function disability regain their independence.
The MyHand™ System is:

Intelligent
Smart device built on intelligent and unique clinical protocols for targeted hand therapy.

Connected
IoMT-based internet-connected device with built-in IRegained app store to access engaging hand-rehab games.

Neuroplastic
Intelligent algorithms developed through deep scientific research on leveraging neuroplasticity and applying it for motor control recovery.

Built for Tele-Rehab
Integral telemedicine platform for remote rehabilitation in the comfort of patient’s home.

Effective
Demonstrated clinical efficacy in recovery of hand function.

Affordable
Affordable system with attractive financing and leasing options.
We offer a compelling value proposition for customers across the full continuum of rehabilitation care in all settings. There is 4-5 month pay back period and strong ROI for clinics, rehab hospitals and long term-care institutions. We also have other models like Lease, Financing and HaaS available to rehabilitation centers and service providers for rapidly adopting this superior technology and demonsrating better value and outcoms for their patients.
Further, COVID-19 has set a trend for expansion of remote rehabilitation and monitoring market, and IRegained's MyHand™ System was designed to cater with this setting.
MyHand™ System is already FDA registered under Class I (lower regulatory barrier = faster market development) and patent protected in US and China and patents pending in the EU, Canada and India (Strong IP = higher competitive barrier).




















OUR TREMENDOUS ACHIEVEMENTS SINCE SEED FUNDING
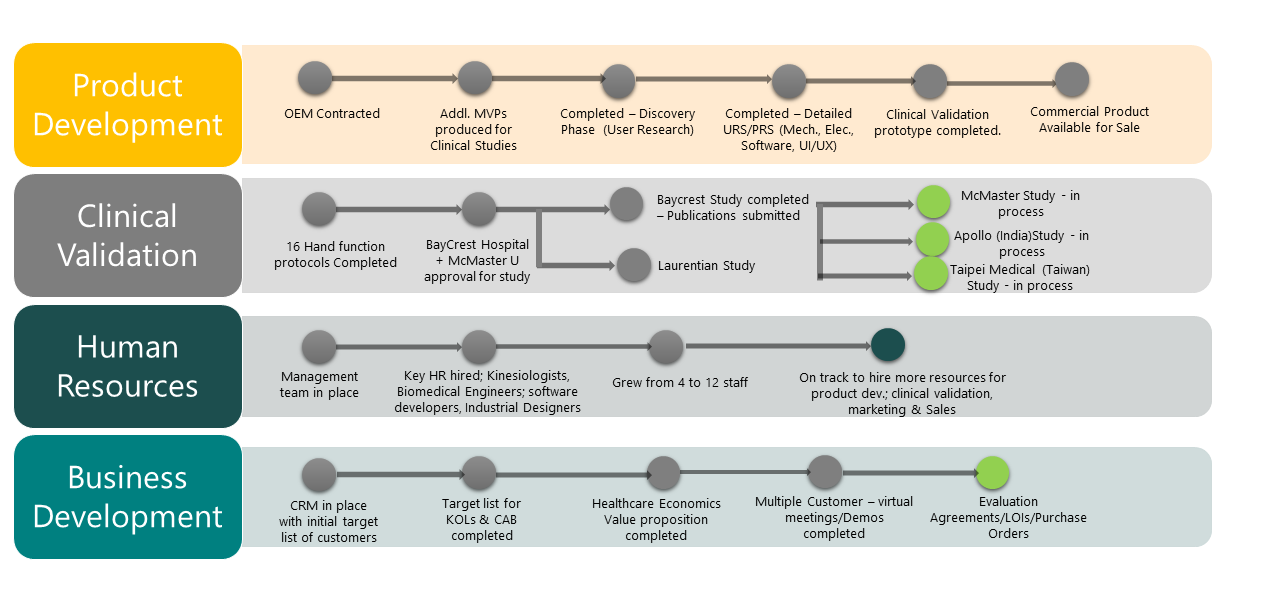

Octane Launchpad Alumni, Irvine, CA, USA -- Accelerator

SoCal Bio, CA, USA – Incubator

Canadian Technology Accelerator (CTA Graduate) – Accelerator Program

University City Science Center & Launch Lane Accelerator, Philadelphia, PA, USA – Accelerator

Ionic Mechatronics, Sudbury, ON, Canada – Hardware Development Partner

Synaptic Technologies, Sudbury, ON, Canada – Software Development Partner

NORCAT, Sudbury, ON, Canada -- Incubator

Foundry, Innovation Centre at Laurentian University, Sudbury, ON, Canada - Incubator

Laurentian University, Sudbury, ON, Canada – Academic Research Partner

Cambrian College, Sudbury, ON, Canada -– Academic Research Partner

Health Sciences North and HSNRI, Sudbury, ON, Canada – Clinical Research Partner

Baycrest Hospital, Toronto, ON, Canada

McMaster University, Sudbury, ON, Canada - – Clinical Research Partner

Sunnybrook Hospital, Toronto, ON, Canada -- – Clinical Research Partner

Sheba Medical Center, Tel Aviv, Israel - – Clinical Research Partner

University of Texas, San Antonio, Texas, USA - – Clinical Research Partner

Christian Medical College & Hospital (CMC&H), Vellore, TN, India - Academic & Clinical Research Partner

Cerner India Pvt. Ltd., Bangalore, India – Advanced Technology Development & R&D Partner

Taipei Medical University, Taipei, Taiwan - Clinical Research Partner

Apollo Hospitals, Hyderabad, India - Clinical Research Partner

i2i, Taipei, Taiwan - Incubator and Industry Partner

BE Accelerator, Taipei, Taiwan - Incubator

Wistron, Taipei, Taiwan - APAC Strategic Distribution Partner

Northern Ontario School of Medicine, Sudbury, ON, Canada - Academic & Clinical Research Partner

TMU Biomed Accelerator, Taipei, Taiwan - Incubator

Tel Aviv Univeristy, Tel Aviv, Israel - Academic Research Partner

University of Texas Health Sciences Center, San Antonio, TX, USA - Academic Research Partner
OUR ADVISOR

Dr. Jocelyn Harris (Chair, CAB)
Associate Professor, McMaster University

Dr. Julie Vaughan Graham
Physical Therapist, Adjunct Lecturer, University of Toronto.

Dr. Yafi Levanon
Occupational Therapist, Tel Aviv University, Sheba Medical Centre, Israel

Dr. Jed Meltzer
Neuroscientist, Baycrest Hospital, University of Toronto

Dr. Shoba Moses
Clinical Occupational Therapist, Dallas, USA

Dr. Michael Franklyn
Prof. Northern Ontario School of Medicine

Peter Dal Bianco
Sudbury, ON Canada

Neelima Firth
Thousand Oaks, CA, USA

David Hood
Long Beach, CA, USA

Rav Sheth
Boston, MA USA

Chad Pallopson
Toronto, ON Canada

Dr. Dennis Reich
Sudbury, ON Canada
JOIN US ON THIS AMAZING JOURNEY
Our Series A Round is currently open. Contact us to learn more.
For this round, IRegained is seeking a total raise of USD $5,000,000 /CAD $ 8,000,000 to deploy resources for marketing, sales, product manufacturing and comemrcialization activities.
The funds will allow us to reach the following milestones:
Manufacture our FDA Registered, commercial MyHand™ System
Launch and distribute our MyHand™ System to all our customer leads and develop new customers through various early adopter and Loan-to-Own Programs
Hire key resources to market and sell the product in USA and Canada
IRegained is on a mission to help 100 million stroke survivors regain their hand function.
We are strategically poised to serve a large, poorly met market that has yet to find the solution they needed by stroke patients to live a healthier, more functional life.
We have achieved significant milestones, despite odds and challenges from COVID-19.
And with your financial support, we can do so much more.
For more information on this investment opportunity, please contact:
Vineet Johnson, CEO